Get Full Access with a FREE Account
- Latest medical news
- Peer-reviewed clinical content
- Conference coverage
- Free CME/CE
- Quizzes to test your knowledge
- Research summaries
Latest News
April 16, 2024
Sugar Substitutes Satisfy Appetite, Blunt Insulin Response
April 16, 2024
MDedge Internal Medicine
April 16, 2024
Children With ASD May Have Earlier Onset of Suicidal Thoughts, Behaviors
April 16, 2024
MDedge Pediatrics
April 16, 2024
The Rise of Positive Psychiatry (and How Pediatrics Can Join the Effort)
April 16, 2024
MDedge Pediatrics
Photo Challenge

April 16, 2024
Progressively Worsening Scaly Patches and Plaques in an Infant
April 16, 2024
MDedge Dermatology
Clinical Reviews

April 8, 2024
Evaluating the Cost Burden of Alopecia Areata Treatment: A Comprehensive Review for Dermatologists
April 8, 2024
MDedge Dermatology
March 5, 2024
Depression As a Potential Contributing Factor in Hidradenitis Suppurativa and Associated Racial Gaps
March 5, 2024
MDedge Dermatology

January 30, 2024
Impact of Ketogenic and Low-Glycemic Diets on Inflammatory Skin Conditions
January 30, 2024
MDedge Dermatology
January 30, 2024
Expanding the Psoriasis Framework: Immunopathogenesis and Treatment Updates
January 30, 2024
MDedge Dermatology
Coronavirus Updates
April 5, 2024
Study Shows Nirmatrelvir–Ritonavir No More Effective Than Placebo for COVID-19 Symptom Relief
April 5, 2024
MDedge Infectious Disease
April 4, 2024
The ED Sailed Smoothly in the Early COVID-19 Days
April 4, 2024
MDedge Infectious Disease
March 28, 2024
No Increased Stroke Risk After COVID-19 Bivalent Vaccine
March 28, 2024
MDedge Infectious Disease
March 26, 2024
Severe Flu Confers Higher Risk for Neurologic Disorders Versus COVID
March 26, 2024
MDedge Infectious Disease
Business of Medicine
April 16, 2024
Family Physician Burnout Rates Remain Stable, But Few Seek Help
April 16, 2024
MDedge Family Medicine
April 16, 2024
Working From Home: Doctors’ Options Are Not Limited to Classic Telemedicine
April 16, 2024
MDedge Internal Medicine
April 15, 2024
How Medicare Reimbursement Trends Could Affect Breast Surgeries
April 15, 2024
MDedge Hematology and Oncology
April 15, 2024
No Routine Cancer Screening Option? New MCED Tests May Help
April 15, 2024
AACR: American Association for Cancer Research
MDedge Hematology and Oncology
April 15, 2024
Are You Ready for AI to Be a Better Doctor Than You?
April 15, 2024
MDedge Internal Medicine
Conference Coverage
April 16, 2024
One-Minute Speech Test Could Help Assess Dementia Risk
April 16, 2024
MDedge Neurology

April 16, 2024
Blood Test Shows Promise for Improving CRC Screening
April 16, 2024
AACR: American Association for Cancer Research
MDedge Hematology and Oncology
April 16, 2024
Positive Results for Intranasal Oxytocin in Adults With Autism
April 16, 2024
MDedge Neurology
April 16, 2024
Prominent Researcher Describes Pivot From ALS Treatment to Prevention
April 16, 2024
AAN: American Academy of Neurology
MDedge Neurology
April 15, 2024
How New ICI Combos Change Bladder Cancer Management
April 15, 2024
MDedge Hematology and Oncology
Medicine's Lighter Side

June 15, 2023
The road to weight loss is paved with collusion and sabotage
June 15, 2023
MDedge Internal Medicine
Perspectives
April 16, 2024
Meat Linked to Higher Erectile Dysfunction Risk
April 16, 2024
MDedge Internal Medicine
April 12, 2024
‘No Pulse’: An MD’s First Night Off in 2 Weeks Turns Grave
April 12, 2024
MDedge Internal Medicine
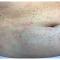
April 12, 2024
A 30-Year-Old White Female Presented With a 4-Month History of Scaly, Erythematous Patches and Plaques on Her Trunk and Extremities
April 12, 2024
MDedge Dermatology
Clinical Edge Journal Scan
April 16, 2024
Sugar Substitutes Satisfy Appetite, Blunt Insulin Response
April 16, 2024
MDedge Internal Medicine
April 16, 2024
Children With ASD May Have Earlier Onset of Suicidal Thoughts, Behaviors
April 16, 2024
MDedge Pediatrics

April 16, 2024
The Rise of Positive Psychiatry (and How Pediatrics Can Join the Effort)
April 16, 2024
MDedge Pediatrics