Get Full Access with a FREE Account
- Latest medical news
- Peer-reviewed clinical content
- Conference coverage
- Free CME/CE
- Quizzes to test your knowledge
- Research summaries
Latest News
May 8, 2024
Physical Activity Protective Against Inflammatory Bowel Disease, Meta-Analysis Shows
May 8, 2024
MDedge Internal Medicine
May 8, 2024
National Mine Safety Group Issues Rule to Reduce Silica Exposure
May 8, 2024
CHEST Physician
May 8, 2024
Satisfactory Results, Less Pain When Surface Anesthesia Used with Thermomechanical Fractional Injury Therapy
May 8, 2024
ASLMS: American Society for Laser Medicine and Surgery
MDedge Dermatology
Photo Challenge
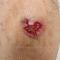
April 29, 2024
Chronic Cribriform Ulcerated Plaque on the Left Calf
April 29, 2024
MDedge Dermatology

April 16, 2024
Progressively Worsening Scaly Patches and Plaques in an Infant
April 16, 2024
MDedge Dermatology
Clinical Reviews
May 6, 2024
An Update on Cutaneous Angiosarcoma Diagnosis and Treatment
May 6, 2024
MDedge Dermatology

April 25, 2024
Dermatologic Care for Refugees: Effective Management of Scabies and Pediculosis
April 25, 2024
MDedge Dermatology

April 8, 2024
Evaluating the Cost Burden of Alopecia Areata Treatment: A Comprehensive Review for Dermatologists
April 8, 2024
MDedge Dermatology
March 5, 2024
Depression As a Potential Contributing Factor in Hidradenitis Suppurativa and Associated Racial Gaps
March 5, 2024
MDedge Dermatology
Coronavirus Updates
April 24, 2024
Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers
April 24, 2024
MDedge Infectious Disease
April 18, 2024
COVID Vaccinations Less Prevalent in Marginalized Patients
April 18, 2024
MDedge Infectious Disease
April 18, 2024
4 Years In, a Sobering Look at Long COVID Progress
April 18, 2024
MDedge Infectious Disease
April 5, 2024
Study Shows Nirmatrelvir–Ritonavir No More Effective Than Placebo for COVID-19 Symptom Relief
April 5, 2024
MDedge Infectious Disease
Business of Medicine

May 8, 2024
Consider a Four-Step Approach to Shared Decision-Making in Pediatric Dermatology
May 8, 2024
AAD: American Academy of Dermatology
MDedge Dermatology
May 7, 2024
Do Health-Related Social Needs Raise Mortality Risk in Cancer Survivors?
May 7, 2024
MDedge Hematology and Oncology
May 7, 2024
Pediatric Clinic Doubles Well-Visit Data Capture by Going Completely Paperless
May 7, 2024
PAS: Pediatric Academic Societies
MDedge Pediatrics
May 6, 2024
Cervical Cancer Screening: US Clinicians Unclear About Best Practices
May 6, 2024
MDedge Hematology and Oncology

May 3, 2024
Prospect of Better Hours, Less Burnout Fuels Locum Tenens
May 3, 2024
MDedge Internal Medicine
Conference Coverage

May 8, 2024
Satisfactory Results, Less Pain When Surface Anesthesia Used with Thermomechanical Fractional Injury Therapy
May 8, 2024
ASLMS: American Society for Laser Medicine and Surgery
MDedge Dermatology

May 8, 2024
Consider a Four-Step Approach to Shared Decision-Making in Pediatric Dermatology
May 8, 2024
AAD: American Academy of Dermatology
MDedge Dermatology

May 7, 2024
Knee Osteoarthritis Trials Show Promising Results for Several Novel Injectables
May 7, 2024
OARSI: OsteoArthritis Research Society International
MDedge Internal Medicine
May 7, 2024
The Inconsistency of Preparticipation Sports Evaluations Raises Issues About Their Utility
May 7, 2024
PAS: Pediatric Academic Societies
MDedge Pediatrics

May 7, 2024
Neutrophils Take Center Stage in Growing Understanding of Colchicine’s Role in Treating Atherosclerotic Cardiovascular Disease
May 7, 2024
MDedge Cardiology
Medicine's Lighter Side

June 15, 2023
The road to weight loss is paved with collusion and sabotage
June 15, 2023
MDedge Internal Medicine
Perspectives
May 7, 2024
Comment on “Skin Cancer Screening: The Paradox of Melanoma and Improved All-Cause Mortality”
May 7, 2024
MDedge Dermatology
Clinical Edge Journal Scan
May 8, 2024
Physical Activity Protective Against Inflammatory Bowel Disease, Meta-Analysis Shows
May 8, 2024
MDedge Internal Medicine
May 8, 2024
National Mine Safety Group Issues Rule to Reduce Silica Exposure
May 8, 2024
CHEST Physician

May 8, 2024
Satisfactory Results, Less Pain When Surface Anesthesia Used with Thermomechanical Fractional Injury Therapy
May 8, 2024
ASLMS: American Society for Laser Medicine and Surgery
MDedge Dermatology







